Showing posts with label Metabolism. Show all posts
Showing posts with label Metabolism. Show all posts
Tuesday, May 3
Hybernation in Space
Sending humans virtually anywhere in space beyond the Moon pushes logistics of health, food, and psychology to limits we're only just beginning to grasp.
A staple solution to these problems in science fiction is to simply put the void-travelers to bed for a while. In a sleep-like state akin to hibernation or torpor, metabolism drops, and the mind is spared the boredom of waiting out endless empty hours.
Unlike faster-than-light travel and wormholes, the premise of putting astronauts into a form of hibernation feels like it's within grasp. Enough so that even the European Space Agency is seriously looking into the science behind it.
Implications of a new study by a trio of researchers from Chile now reveal a mathematical hurdle to turning the potential of long-term human stasis into reality, one that might mean it's as forever beyond our reach.
Roberto F. Nespolo and Carlos Mejias from the Millennium Institute for Integrative Biology and Francisco Bozinovic from the Pontifical Catholic University of Chile set out to unravel the relationship between body mass and energy expenditure in animals that hibernate.
They discovered a minimum level of metabolism that allows cells to persist under cold, low-oxygen conditions. For relatively heavy animals like us, the energy savings we might expect from entering a deep, hibernation-like state would be negligible.
In fact, we'd probably be better off just napping our days away the old-fashioned way.
The word hibernation often invokes images of a bear tucked away in a den for a long winter's rest. READ MORE...
Thursday, August 19
Thought and Metabolism
To regulate adaptive behaviour, the brain relies on a continuous flow of cognitive and memory-related processes that require a constant energy supply. Weighing around 1,200 grams in women and 1,300 grams in men, on average, the brain consumes around 90 grams, or 340 kilocalories’ worth, of glucose per day, accounting for around half of the body’s glucose demand1,2.
The tight integration of metabolic and cognition-related signals might aid the matching of the brain’s energy supply to its energy needs, by optimizing foraging behaviour and efforts to limit energy expenditure.
The synchronization of glucose supply with brain activity has so far been considered a function of a structure called the hypothalamus, at the base of the brain. Writing in Nature, Tingley et al.3 provide evidence in rats for the role of another brain region, called the hippocampus, which is typically implicated in memory and navigation, in this equation (Fig. 1).
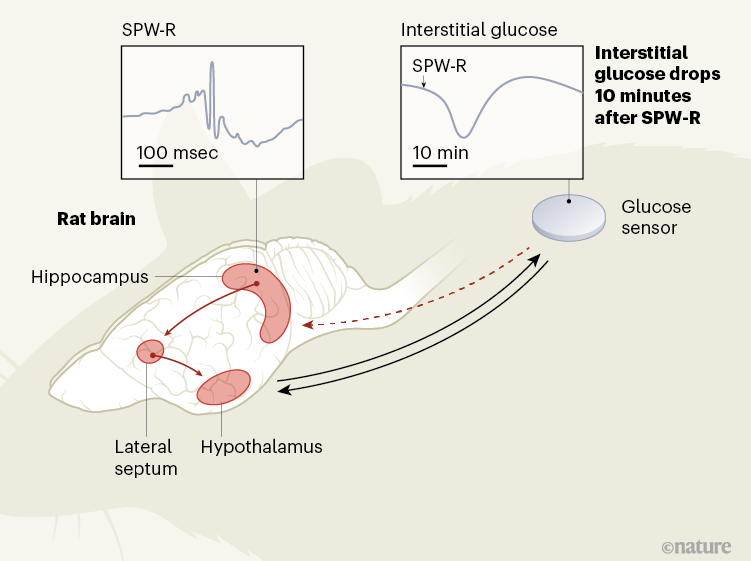
Figure 1 | Brain signals that regulate glucose levels in the body periphery. The hypothalamus in the brain helps to regulate glucose concentrations in the blood and in the interstitial fluid that surrounds cells in the body. This hypothalamic (feedback-mediated) regulation is activated, for example, during stress. Tingley et al.3 provide evidence in rats that another brain structure, the hippocampus, also regulates peripheral glucose concentrations. In the hippocampus, oscillatory patterns — called sharp wave-ripples (SPW-Rs) — emerge in the collective electrical potential across the membranes of neurons. They seem to signal, by way of a region called the lateral septum, to the hypothalamus to produce dips in interstitial glucose concentration about 10 minutes later. The feedback mechanism in this regulatory loop is unknown (dashed arrow). Given that hippocampal SPW-Rs are a hallmark of the reprocessing of previous experiences, they might thus control the brain’s energy supply during a ‘thought-like’ mode.
The hippocampus receives many types of sensory and metabolic information, and projections from neuronal cells in the hippocampus extend to various parts of the brain, including the hypothalamus. Thus, the hippocampus might indeed represent a hub in which metabolic signals are integrated with cognitive processes3.

Figure 1 | Brain signals that regulate glucose levels in the body periphery. The hypothalamus in the brain helps to regulate glucose concentrations in the blood and in the interstitial fluid that surrounds cells in the body. This hypothalamic (feedback-mediated) regulation is activated, for example, during stress. Tingley et al.3 provide evidence in rats that another brain structure, the hippocampus, also regulates peripheral glucose concentrations. In the hippocampus, oscillatory patterns — called sharp wave-ripples (SPW-Rs) — emerge in the collective electrical potential across the membranes of neurons. They seem to signal, by way of a region called the lateral septum, to the hypothalamus to produce dips in interstitial glucose concentration about 10 minutes later. The feedback mechanism in this regulatory loop is unknown (dashed arrow). Given that hippocampal SPW-Rs are a hallmark of the reprocessing of previous experiences, they might thus control the brain’s energy supply during a ‘thought-like’ mode.
The hippocampus receives many types of sensory and metabolic information, and projections from neuronal cells in the hippocampus extend to various parts of the brain, including the hypothalamus. Thus, the hippocampus might indeed represent a hub in which metabolic signals are integrated with cognitive processes3.
To examine this possibility, Tingley and colleagues recorded oscillatory patterns called sharp wave-ripples (SPW-Rs), reflecting changes in electrical potential across the cell membranes of neuronal-cell ensembles in the hippocampi of rats. They did this while using a sensor inserted under the skin of the animals’ backs to continuously measure glucose levels in the interstitial fluid surrounding the cells there. READ MORE
Tuesday, August 17
Our Metabolism
A new international study counters the common belief that our metabolism inevitably declines during our adult lives. Well, not until we’re in our 60s, anyway.
Researchers found that metabolism peaks around age 1, when babies burn calories 50 percent faster than adults, and then gradually declines roughly 3 percent a year until around age 20.
From there, metabolism plateaus until about age 60, when it starts to slowly decline again, by less than 1 percent annually, according to findings published Thursday in the journal Science.
To tease out the specific impact of age on metabolism, the researchers adjusted for factors such as body size (bigger bodies burn more calories overall than smaller ones) and fat-free muscle mass (muscles burn more calories than fat).
“Metabolic rate is really stable all through adult life, 20 to 60 years old,” said study author Herman Pontzer, an associate professor of evolutionary anthropology at Duke University and author of “Burn,” a new book about metabolism.
To tease out the specific impact of age on metabolism, the researchers adjusted for factors such as body size (bigger bodies burn more calories overall than smaller ones) and fat-free muscle mass (muscles burn more calories than fat).
“Metabolic rate is really stable all through adult life, 20 to 60 years old,” said study author Herman Pontzer, an associate professor of evolutionary anthropology at Duke University and author of “Burn,” a new book about metabolism.
“There's no effect of menopause that we can see, for example. And you know, people will say, 'Well when I hit 30 years old, my metabolism fell apart.' We don't see any evidence for that, actually.”
Pontzer and colleagues studied a database of more than 6,400 people, ages 8 days to 95 years, from 29 countries worldwide who had participated in “doubly labeled water” tests.
Pontzer and colleagues studied a database of more than 6,400 people, ages 8 days to 95 years, from 29 countries worldwide who had participated in “doubly labeled water” tests.
With this method, individuals drink water in which some of the hydrogen and oxygen have been replaced with isotopes of these elements that can be traced in urine samples. READ MORE
Subscribe to:
Comments (Atom)

